Sunday, December 16, 2012
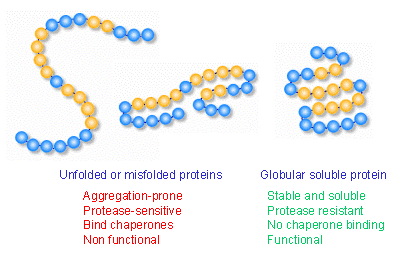
Thermodynamics and Protein Folding
Thursday, December 6, 2012
Sugar Solubility and Intake
In exercise drinks such as Gatorade and Powerade, sugar is the main fuel source for the body. These drinks contain simple sugars because they are absorbed into the body faster and can be used as energy faster. Athletes depend on these simple sugars as fuel and they need them for better performance. For example, someone who races in a sport such as swimming or track will use these fast dissolving sugars as a quick burst of energy for a sprint. However, they must monitor their intake of the sugars; they must take the appropriate amount and take it with the correct frequency. When someone drinks Gatorade and the sugars enter their body, they are absorbed into the bloodstream quickly. The pancreas must pump out insulin in order to compensate for the rise in blood sugar, also known as hyperglycemia. But the sugars dissolve so quickly that the actual amount of them is overestimated and there is always insulin left over. This extra insulin is stored as fat in the body, which is bad for the athlete. In order to prevent this from happening, the athlete needs to have a continuous and constant intake of these sugars so that the blood sugar will not spike and there will not be extra insulin left over in the body. Although it is good that these simple sugars can be used as fuel quickly, a lot of athletes make the mistake of ignoring the importance of intake frequency.
This issue is caused because of the simple sugars' solubility properties. Fructose is the most soluble of all the simple sugars. In solutions, like dissolves like, meaning polar compounds will dissolve polar compounds and nonpolar compounds will dissolve nonpolar compounds. Fructose is polar so it will easily dissolve in water, a polar solvent. In addition, it is a smaller compound so it will dissolve faster than a larger one, such as sucrose, a disaccharide.
Wednesday, December 5, 2012
Casein Proteins and Solubility
Milk proteins are some of the best proteins for their nutritional and technological value. Proteins are often a big part of peoples' diets, especially because they provide the essential amino acids and are big sources of nitrogen. Milk proteins have the best nutritional value compared to other proteins because of the high content of essential amino acids. Additionally, the good digestibility can also add to the great nutritional value.
Caseins and whey proteins are the two main protein groups that are found in milk. Caseins are found in 80 % of proteins in most milk, but are isolated from milk by acid. The acid precipitation is performed at a pH of 4.6, which is where caseins precipitate and whey proteins remain soluble. Caseins are often flexible and heat stable proteins.
The other 20 % of proteins in milk is composed of whey protein. Whey proteins are also known as proteins in milk that can remain soluble after acid precipitation, which is often found in milk. The whey protein acid that is used is formally known as acid whey, or informally as sweet whey. Whey proteins are globular proteins that are soluble over a broad pH range.
Most of the time, whey proteins have been considered as a more balanced and superior alternative to casein. The amino acids that are found in whey appear at a higher concentration in whey proteins. Whey proteins are also more similar to human milk, which is why whey proteins are more often known as the replacement for humanized milk production. Another reason as to why caseins are inferior is due to the lower digestion and absorption than found in whey proteins.
In a recent study, protein solubility was measured in the pH range of 2 to 10. A mixture of sodium caseinate and their hydrolysates had minimum solubility at a pH around 4 or 5. They had the highest solubility level in alkalinic pH range, which follows the results of most studies. The results of the study proved that enzymatic hydrolysis improved solubility of their hydrolysates. The increased number of ionizable groups with an increase in hydrophilicity and net charge resulting in hydrolysates promotes hydrolysate-water interaction, thus improving the solubility. The small molecular size also inceases the solubility.
IMF is a major way of knowing whether or not something has a high solubility in water. Remember that, like dissolves like. Using this logic, the polar water would dissolve the most polar protein models. The two main determinants of IMF in a molecule are charge and size. As the charge increases, the solubility increases in water. The smaller the size of the compound, the greater the solubility of the compound in water.
Casein is made of a large amount of residues, which do not interact. There is little structure to a molecule like this. It is also hydrophobic, which makes it less soluble in water. When it is found in suspension in milk for particles, it is held togther by calcium ions. These calcium ions and hydrophobic interactions make up the molecule for casein. The isoelectric point of casein is 4.6. Milk has a pH of 6.6, so casein has a negative charge in milk, so a purified version of casein protein is insoluble in water.
Caseins and whey proteins are the two main protein groups that are found in milk. Caseins are found in 80 % of proteins in most milk, but are isolated from milk by acid. The acid precipitation is performed at a pH of 4.6, which is where caseins precipitate and whey proteins remain soluble. Caseins are often flexible and heat stable proteins.
The other 20 % of proteins in milk is composed of whey protein. Whey proteins are also known as proteins in milk that can remain soluble after acid precipitation, which is often found in milk. The whey protein acid that is used is formally known as acid whey, or informally as sweet whey. Whey proteins are globular proteins that are soluble over a broad pH range.
Most of the time, whey proteins have been considered as a more balanced and superior alternative to casein. The amino acids that are found in whey appear at a higher concentration in whey proteins. Whey proteins are also more similar to human milk, which is why whey proteins are more often known as the replacement for humanized milk production. Another reason as to why caseins are inferior is due to the lower digestion and absorption than found in whey proteins.
In a recent study, protein solubility was measured in the pH range of 2 to 10. A mixture of sodium caseinate and their hydrolysates had minimum solubility at a pH around 4 or 5. They had the highest solubility level in alkalinic pH range, which follows the results of most studies. The results of the study proved that enzymatic hydrolysis improved solubility of their hydrolysates. The increased number of ionizable groups with an increase in hydrophilicity and net charge resulting in hydrolysates promotes hydrolysate-water interaction, thus improving the solubility. The small molecular size also inceases the solubility.
IMF is a major way of knowing whether or not something has a high solubility in water. Remember that, like dissolves like. Using this logic, the polar water would dissolve the most polar protein models. The two main determinants of IMF in a molecule are charge and size. As the charge increases, the solubility increases in water. The smaller the size of the compound, the greater the solubility of the compound in water.
Casein is made of a large amount of residues, which do not interact. There is little structure to a molecule like this. It is also hydrophobic, which makes it less soluble in water. When it is found in suspension in milk for particles, it is held togther by calcium ions. These calcium ions and hydrophobic interactions make up the molecule for casein. The isoelectric point of casein is 4.6. Milk has a pH of 6.6, so casein has a negative charge in milk, so a purified version of casein protein is insoluble in water.
Tuesday, December 4, 2012
Review of Electrolyte Blog Post by a Nutritionist
Original blog post: http://rackupvitamind.blogspot.com/2012/11/electrolytes.html
If you added this to your blog, it would help get your point across and help add to the content section:
Electrolytes are lost during exercise if one becomes dehydrated. One way to avoid electrolyte imbalance is to drink water during exercise. The amount and frequency needed will depend on the severity of the activity and temperature of the atmosphere. For example, you will require more fluids to remain hydrated if you are exercising outdoors in 80 degree temperatures versus exercising in an air-conditioned gym. As long as you maintain an adequate intake of fluids during exercise, your electrolytes will not become depleted.
However if insufficient fluids are no consumed, and as a result, electrolytes are lost; they will need to be replaced. One quick way is with a sports drink such as Gatorade or Powerade. These are sugar-based drinks that contain sodium and potassium, the minerals that are lost during exercise when dehydration occurs. The sugar (carbohydrates) in these drinks act as a fuel source for the body. This is beneficial during long endurance exercise, but it will metabolize rapidly and thus require frequent replenishment. The sole source of energy in these drinks is sugar, and for someone concerned about their sugar intake, they should consider keeping themselves well hydrated with water during their exercise.
Drinks that contain protein and/or amino acids are not usually meant for consumption to maintain/replace electrolytes. They are frequently consumed, although not necessary, to build/maintain muscle. Someone who consumes an adequate amount of good quality protein in their daily diet will not need to consume supplemental protein drinks. However, if one has a desire to do so, they should make sure that it contains good quality protein composed of 9 essential amino acids.
One easy source of fluids, energy, electrolytes, and protein is milk. Although it is not practical for consumption during exercise due to its microbiological spoilage properties, it is a complete food source to consume after exercising.
Written by Deborah A. Gallucio, M.S., R.D.
Ms. Gallucio received her Bachelor of Science in Foods and Nutrition from Montclair State University and her Master of Science in Nutrition and Public Health from Columbia University. She is a registered dietitian with the American Dietetic Association and has been in private practice for 31 years.
If you added this to your blog, it would help get your point across and help add to the content section:
Electrolytes are lost during exercise if one becomes dehydrated. One way to avoid electrolyte imbalance is to drink water during exercise. The amount and frequency needed will depend on the severity of the activity and temperature of the atmosphere. For example, you will require more fluids to remain hydrated if you are exercising outdoors in 80 degree temperatures versus exercising in an air-conditioned gym. As long as you maintain an adequate intake of fluids during exercise, your electrolytes will not become depleted.
However if insufficient fluids are no consumed, and as a result, electrolytes are lost; they will need to be replaced. One quick way is with a sports drink such as Gatorade or Powerade. These are sugar-based drinks that contain sodium and potassium, the minerals that are lost during exercise when dehydration occurs. The sugar (carbohydrates) in these drinks act as a fuel source for the body. This is beneficial during long endurance exercise, but it will metabolize rapidly and thus require frequent replenishment. The sole source of energy in these drinks is sugar, and for someone concerned about their sugar intake, they should consider keeping themselves well hydrated with water during their exercise.
Drinks that contain protein and/or amino acids are not usually meant for consumption to maintain/replace electrolytes. They are frequently consumed, although not necessary, to build/maintain muscle. Someone who consumes an adequate amount of good quality protein in their daily diet will not need to consume supplemental protein drinks. However, if one has a desire to do so, they should make sure that it contains good quality protein composed of 9 essential amino acids.
One easy source of fluids, energy, electrolytes, and protein is milk. Although it is not practical for consumption during exercise due to its microbiological spoilage properties, it is a complete food source to consume after exercising.
Written by Deborah A. Gallucio, M.S., R.D.
Ms. Gallucio received her Bachelor of Science in Foods and Nutrition from Montclair State University and her Master of Science in Nutrition and Public Health from Columbia University. She is a registered dietitian with the American Dietetic Association and has been in private practice for 31 years.
Monday, December 3, 2012
Proteins in Solutions
Proteins can often be folded into their native form, which helps determine several chemical, physical, and biological properties. These folded proteins are so small that they can actually diffuse or rotate. Proteins have a huge variety of solubility inside of different solutions. Cytoplasmic proteins have about a 35 % in an aqueous solution. Structural proteins are basically insoluble along with proteins that are present in membranes. The solubility of a protein in water is determined by its free energy when surrounded by aqueous solvent relative to in amorphous or solid conditions.
Protein molecules have a lot of interactions with their solvents. These interactions are primarily determined by the solution's surface. There are a lot of favorable interactions with water caused by charged and polar groups in protein chains. The solubility of a globular protein increases as the pH value of the solution mixture goes further and further from the normal pH. If a protein is not very soluble, detergents can be put into the solutions in order to make the proteins unfold.
Proteins in an aqueous solution are tightly bounded by a hydration layer. This hydration layer is more bounded with less mobile that is found in bulk water. It has a 10 % greater density and 15 % greater heat capacity. The interactions between the solvent water and the solute, protein would occur in the charged groups on the surface of the protein, the polar groups on the surface of the protein, and the monolayer around the protein molecule.
Instead of using a detergent, another way of increasing the solubility of a protein would be to increase the ionic strength at low values. This is also known as "salting-in". At higher salt concentrations, the solubility level of a protein would decrease; another way of saying this would be "salt-out". To increase the surface tension of the salt, then the added salt must go up. The higher the surface tension of the salt, the higher the IMF. Therefore, in water, it would be more polar, resulting in a more soluble solution.
Protein molecules have a lot of interactions with their solvents. These interactions are primarily determined by the solution's surface. There are a lot of favorable interactions with water caused by charged and polar groups in protein chains. The solubility of a globular protein increases as the pH value of the solution mixture goes further and further from the normal pH. If a protein is not very soluble, detergents can be put into the solutions in order to make the proteins unfold.
Proteins in an aqueous solution are tightly bounded by a hydration layer. This hydration layer is more bounded with less mobile that is found in bulk water. It has a 10 % greater density and 15 % greater heat capacity. The interactions between the solvent water and the solute, protein would occur in the charged groups on the surface of the protein, the polar groups on the surface of the protein, and the monolayer around the protein molecule.
Subscribe to:
Comments (Atom)